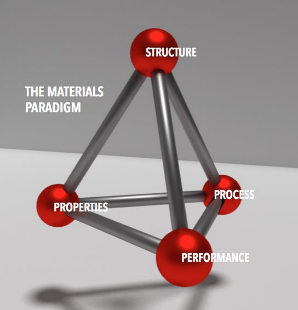
While the development of medical and pharmaceutical devices not only takes time and money, it is also highly regulated. Selecting the right choice of material and process is essential in order to meet compliance regulations, ensure certification processes and reproducibility, and most importantly, provide a high quality product.
Dr. Bhatt will discuss several medical grade polymer materials which have been developed, exhibiting unique characteristics that offer extraordinary benefits over non-polymeric materials in the modern healthcare system. Additionally, these materials comply with global regulatory requirements that ensure they maintain their integrity without causing secondary and/or long-term complications in the patient. Using a specific case study, “Material Development for a Hip Implant Application”, he will discuss the development process and requirements to wade through the myriad of stages In addition, the following 5 criteria must be evaluated thoroughly: (1) product design and application specifications; (2) material properties and consideration; (3) cost; (4) regulatory and handling aspects; and (5) manufacturing and quality plan(s).
Images are licensed under Creative Commons License.

