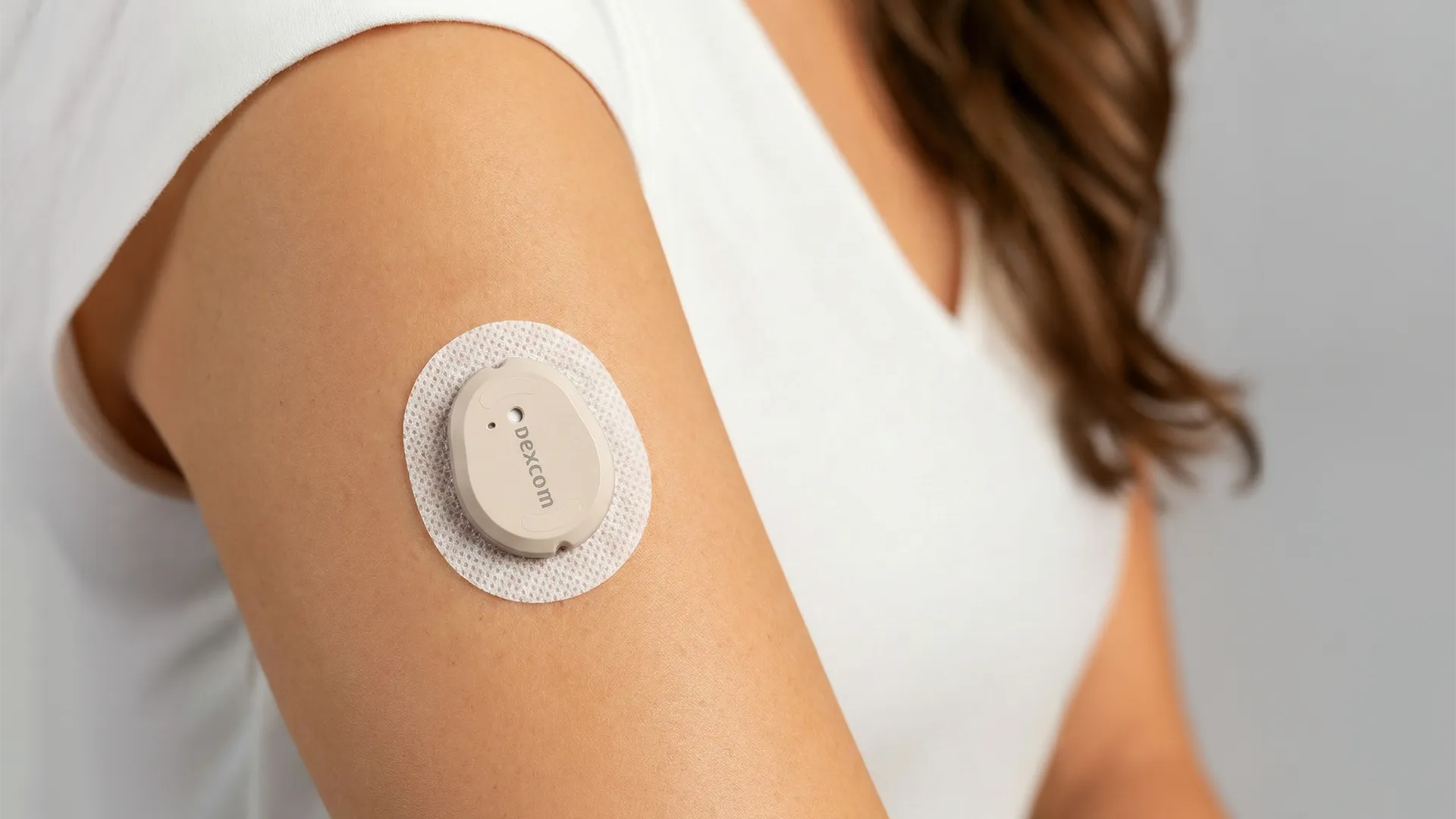
Continuous Glucose Monitoring
Practice Areas
Challenge
For the past 10 years, Triple Ring has assisted pioneering Continuous Glucose Monitor (CGM) manufacturer, Dexcom, in responding to customer demands and mounting competition. Dexcom has engaged Triple Ring at critical points in the company’s history as market forces required rapid evolution of product designs to address usability, reliability, and manufacturability. Triple Ring contributed critical analytical expertise and design inputs while simultaneously enabling new features and increasing manufacturing yields at high volume. Over time, Dexcom and Triple Ring collaborated seamlessly and efficiently to shorten time to market for 2 generations of CGM devices.
Outcomes
Dexcom, together with Triple Ring, developed and optimized a fully automated one-touch deployment mechanism for their award-winning G6 platform. Five years later, the G7 successor was released with a completely new applicator that combined deployment of the wearable and sensor into a single step. Triple Ring executed a mutually supportive analytical and empirical strategy to investigate design issues, resolving both undesirable behavior and generating real-life data for improving model accuracy. This approach maximized learning at each design cycle, ensured a robust and reliable product, and expedited time to market.
Value Propositions
- Deep experience in user-centered design and design for manufacture (DfM)
- Deep expertise in system-level analysis, mechanism analysis and finite element simulation
- Proven success in test development and fixture design for capturing complex phenomena
- Rapid execution of benchtop and preclinical testing
- Proven processes and approaches that significantly reduce time to market
Continuous Glucose Monitoring
Challenge
For the past 10 years, Triple Ring has assisted pioneering Continuous Glucose Monitor (CGM) manufacturer, Dexcom, in responding to customer demands and mounting competition. Dexcom has engaged Triple Ring at critical points in the company’s history as market forces required rapid evolution of product designs to address usability, reliability, and manufacturability. Triple Ring contributed critical analytical expertise and design inputs while simultaneously enabling new features and increasing manufacturing yields at high volume. Over time, Dexcom and Triple Ring collaborated seamlessly and efficiently to shorten time to market for 2 generations of CGM devices.
Outcomes
Dexcom, together with Triple Ring, developed and optimized a fully automated one-touch deployment mechanism for their award-winning G6 platform. Five years later, the G7 successor was released with a completely new applicator that combined deployment of the wearable and sensor into a single step. Triple Ring executed a mutually supportive analytical and empirical strategy to investigate design issues, resolving both undesirable behavior and generating real-life data for improving model accuracy. This approach maximized learning at each design cycle, ensured a robust and reliable product, and expedited time to market.
Value Propositions
- Deep experience in user-centered design and design for manufacture (DfM)
- Deep expertise in system-level analysis, mechanism analysis and finite element simulation
- Proven success in test development and fixture design for capturing complex phenomena
- Rapid execution of benchtop and preclinical testing
- Proven processes and approaches that significantly reduce time to market
Practice Areas

Background
By leveraging Triple Ring’s deep expertise in device development, mechanism analysis, finite-element simulation, system-level modeling, and complex empirical characterization, Dexcom was able to model the effects of manufacturing variability across hundreds of thousands of virtual devices. This approach enabled the identification of unforeseen design sensitivities early in the development process, when the implementation of changes was less costly and impactful to the overall system design. Triple Ring is proud to be a long-term trusted partner for Dexcom as they innovate in diabetes management and improve the quality of life for millions of patients.
Dexcom has been a tremendous partner, and we have thoroughly enjoyed working closely with them for over a decade now. They have a deeply technical team who share Triple Ring’s commitment to engineering rigor and excellence in medical devices and it has served as a great example of how investment in knowledge-driven product development strategies can deliver effective product solutions that positively impact patient lives.
Gabe Chow, Ph.D.

Cable-Free Synthesized ECG
Practice Areas
Challenge
HeartBeam sought to develop a personal, cable-free, and easy-to-use cardiac monitoring solution for in-clinic and at-home use by patients, enabling physicians to assess where vague cardiac symptoms warrant additional diagnostic testing or treatment. Our objective was to create a credit card-sized, ECG recording device that leverages vectorcardiography (VECG) and is integrated with cloud-based software to deliver critical data to physicians in real-time.
Outcomes
The HeartBeam-Triple Ring collaboration produced a personal, cable-free synthesized 12L ECG solution that empowers patients to record synthesized 12L ECG data at home. The device was integrated with a smartphone application to communicate with the HeartBeam Cloud where synthesized 12-lead ECGs are overlaid with baseline data. Physicians are now able to remotely view results and direct next steps for patients in the comfort of their own home. This multidisciplinary and fast-paced program resulted in a 510(k) submission to FDA, and clearance in 2024 for the hardware that leverages vectorcardiography and collects 3D ECG, while the synthesized 12L ECG algorithm is pending FDA clearance.
Value Propositions
- Comprehensive product development, including early R&D, industrial design, product development, manufacturing, and regulatory approval
- Relevant and specific expertise in wearable medical device design
- Regulatory and manufacturing support, including preparation for a 510(k) submission and design transfer
- Variable resources applied just-in-time
Cable-Free Synthesized ECG
Challenge
HeartBeam sought to develop a personal, cable-free, and easy-to-use cardiac monitoring solution for in-clinic and at-home use by patients, enabling physicians to assess where vague cardiac symptoms warrant additional diagnostic testing or treatment. Our objective was to create a credit card-sized, ECG recording device that leverages vectorcardiography (VECG) and is integrated with cloud-based software to deliver critical data to physicians in real-time.
Outcomes
The HeartBeam-Triple Ring collaboration produced a personal, cable-free synthesized 12L ECG solution that empowers patients to record synthesized 12L ECG data at home. The device was integrated with a smartphone application to communicate with the HeartBeam Cloud where synthesized 12-lead ECGs are overlaid with baseline data. Physicians are now able to remotely view results and direct next steps for patients in the comfort of their own home. This multidisciplinary and fast-paced program resulted in a 510(k) submission to FDA, and clearance in 2024 for the hardware that leverages vectorcardiography and collects 3D ECG, while the synthesized 12L ECG algorithm is pending FDA clearance.
Value Propositions
- Comprehensive product development, including early R&D, industrial design, product development, manufacturing, and regulatory approval
- Relevant and specific expertise in wearable medical device design
- Regulatory and manufacturing support, including preparation for a 510(k) submission and design transfer
- Variable resources applied just-in-time
Practice Areas

Background
HeartBeam collaborated with Triple Ring to design their innovative telehealth solution. The joint effort encompassed a five-phase expedited device development program, including device builds for design verification and validation, device packaging, and manufacturing technology transfer. HeartBeam, Inc. (NASDAQ: BEAT) is a medical technology company dedicated to transforming the detection and monitoring of critical cardiac conditions. The Company is creating the first-ever cable-free device capable of collecting ECG signals in 3D, from three non-coplanar directions, and synthesizing the signals into a 12-lead ECG. This platform technology is designed for portable devices that can be used wherever the patient is to deliver actionable heart intelligence. Physicians will be able to identify cardiac health trends and acute conditions and direct patients to the appropriate care – all outside of a medical facility, thus redefining the future of cardiac health management.

Real-Time Tissue Oxygenation Status
Client
ViOptix
Practice Areas
Challenge
ViOptix engaged with Triple Ring Technologies in a co-development partnership to design and develop their Intra.Ox non-invasive, quantitative, handheld tissue oxygenation monitor. Design requirements included real-time tissue monitoring inside the surgical theater with high sensitivity and reliability. Technical challenges faced in the Intra.Ox product development program included:
- Design of an easy-to-use multi-wavelength tissue oximeter
- Smart algorithms to automatically compensate for variable tissue morphologies
- Handheld form factor
- Reusable packaging
- Unlimited readings within a surgery case
Outcomes
The ViOptix Intra.Ox technology received FDA clearance after the company and Triple Ring demonstrated its capability to produce accurate, instantaneous estimation of percent saturated oxygen (St02) without the need for capital equipment or dye injection. The Intra.Ox device is now used at various stages of tissue transfer surgery to positively affect clinical and financial outcomes.
Value Propositions
- Deep, relevant clinical and technical domain knowledge
- Concept to clinical validation and FDA 510k clearance in 18 months
- Complex modeling, including Monte Carlo simulations to accelerate design process
- Urgency to drive value and support fundraising
- Capital efficient, variable resource R&D expertise tailored to the needs of a virtual startup
Real-Time Tissue Oxygenation Status
Challenge
ViOptix engaged with Triple Ring Technologies in a co-development partnership to design and develop their Intra.Ox non-invasive, quantitative, handheld tissue oxygenation monitor. Design requirements included real-time tissue monitoring inside the surgical theater with high sensitivity and reliability. Technical challenges faced in the Intra.Ox product development program included:
- Design of an easy-to-use multi-wavelength tissue oximeter
- Smart algorithms to automatically compensate for variable tissue morphologies
- Handheld form factor
- Reusable packaging
- Unlimited readings within a surgery case
Outcomes
The ViOptix Intra.Ox technology received FDA clearance after the company and Triple Ring demonstrated its capability to produce accurate, instantaneous estimation of percent saturated oxygen (St02) without the need for capital equipment or dye injection. The Intra.Ox device is now used at various stages of tissue transfer surgery to positively affect clinical and financial outcomes.
Value Propositions
- Deep, relevant clinical and technical domain knowledge
- Concept to clinical validation and FDA 510k clearance in 18 months
- Complex modeling, including Monte Carlo simulations to accelerate design process
- Urgency to drive value and support fundraising
- Capital efficient, variable resource R&D expertise tailored to the needs of a virtual startup
Client
ViOptix
Practice Areas
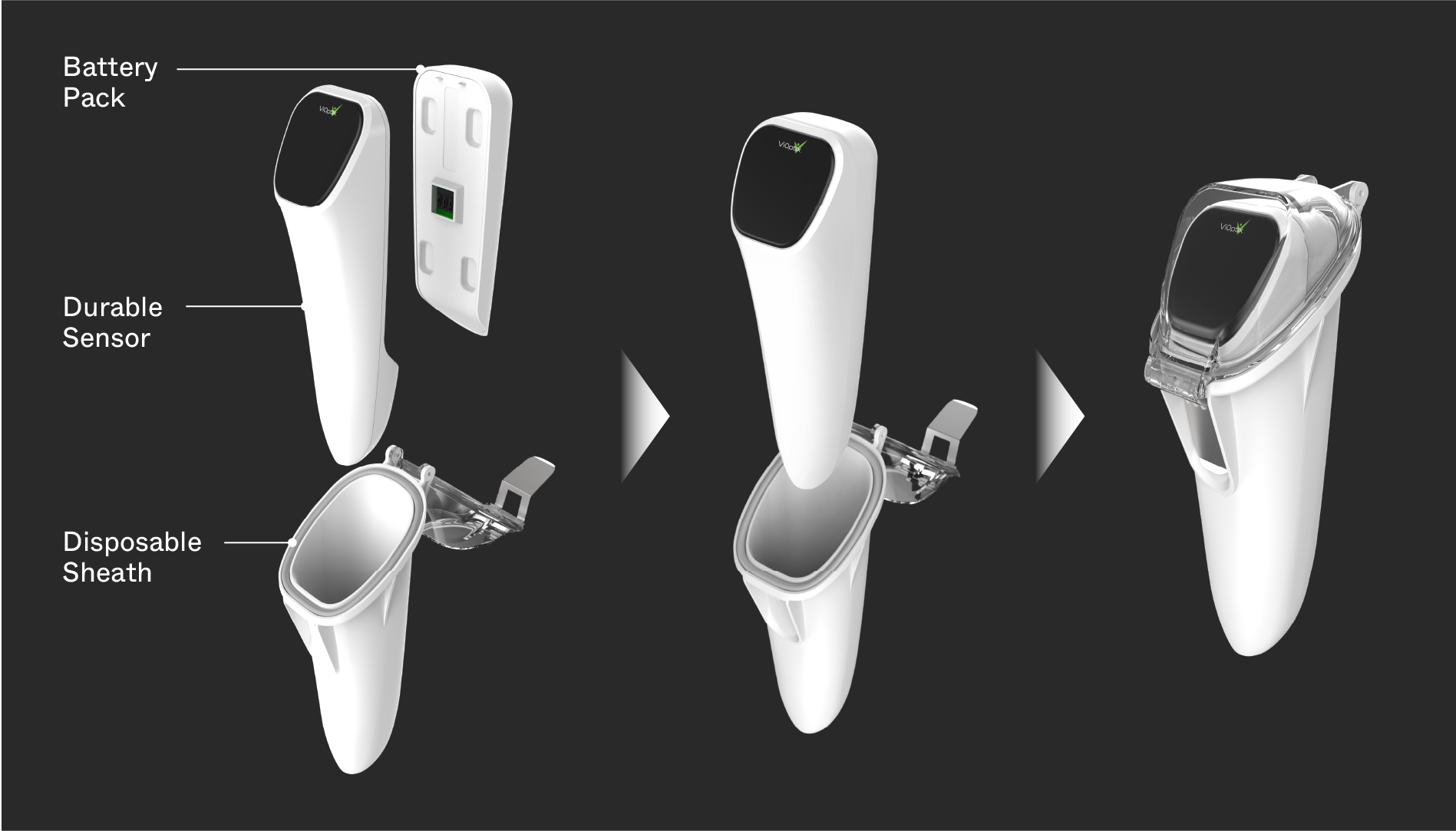


Background
Assessing tissue viability during surgery enables early intervention to improve patient outcomes post-surgery. ViOptix came to Triple Ring seeking solutions for capturing oxygen concentration in resected tissue while mitigating artifacts from surrounding tissue components. The teams worked side-by-side to invent novel solutions at the subsystem and system level, creating a product that promotes faster surgical recovery times, benefitting patients and the hospitals that care for them.
A great thing about working at Triple Ring is that we can both see a wide variety of projects and technologies and develop key technologies that go all the way from concept to treating patients.” The relationship works well for the client also. Scott Coleridge, the ViOptix CEO observes, “Triple Ring has served as our development and engineering group since we decided to go virtual. I don’t think we could have found a higher caliber of scientists and engineers under one roof. They are flexible to work with and have grown with our needs. I would highly recommend them.
ViOptix
Scott Coleridge, (CEO) and Mark Lonsinger, (GM) are family now and we treat ViOptix like it’s our own company,” says Kate Bechtel as she describes ViOptix, a Triple Ring client aiming to improve surgical outcomes through the deployment of technology that can easily measure soft-tissue health in the operating room.
Triple Ring
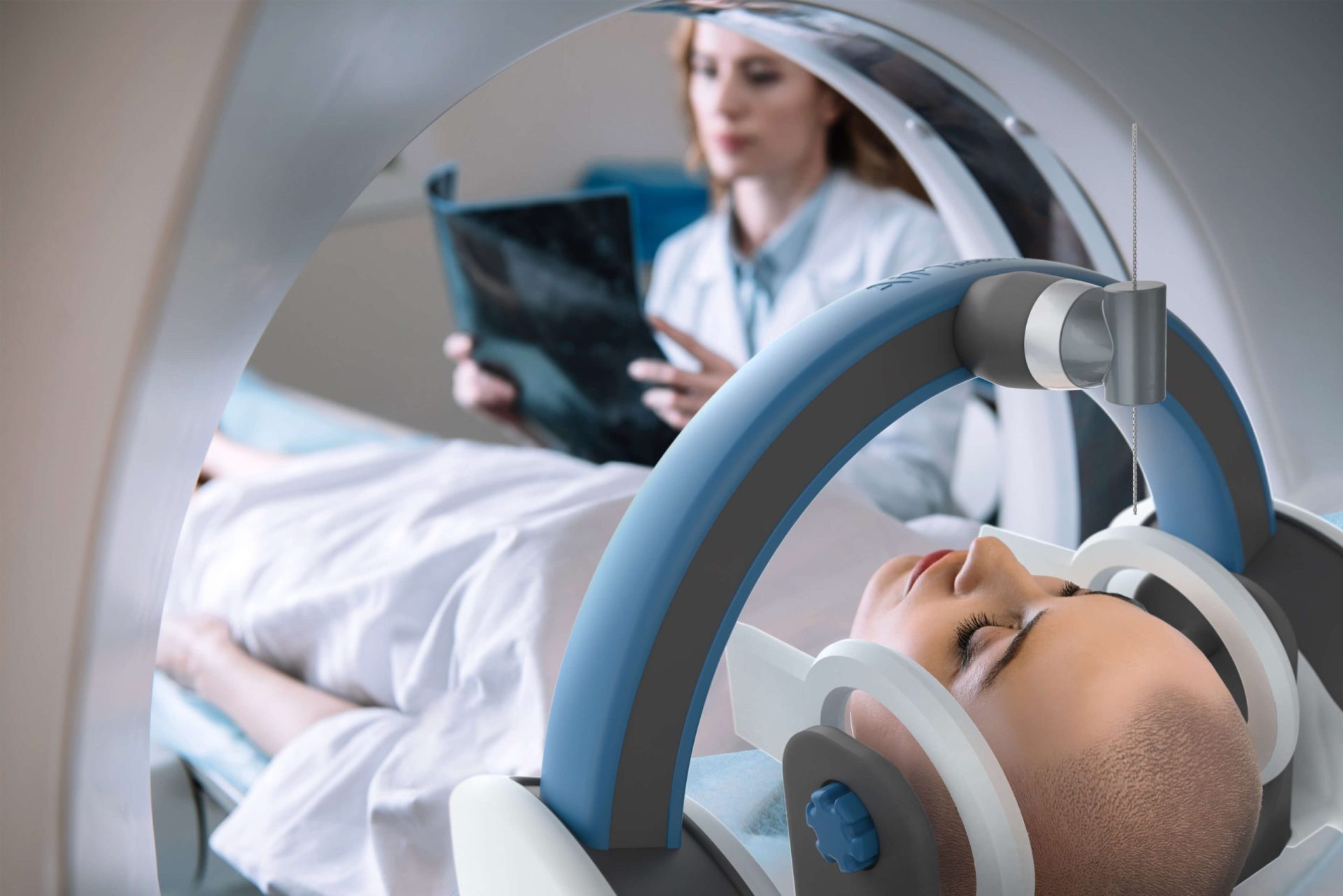
MRI-Compatible Robotics
Client
AiM Medical Robotics
Practice Areas
Challenge
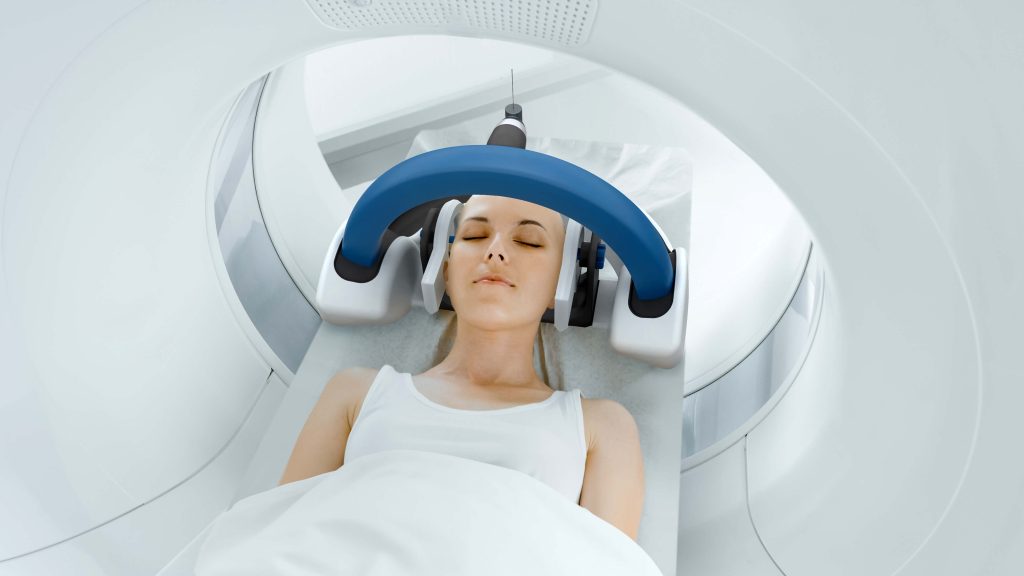
AiM Medical Robotics required a portable robotic system to perform neurosurgery simultaneous with magnetic resonance imaging (MRI). MRI relies on incredibly strong magnetic fields to produce detailed images of the inside of the human body. These strong magnetic fields prohibit the use of ferromagnetic materials that are found in typical robotic hardware. Furthermore, MRI systems feature a small bore around the patient table which severely restricts the form factor of potential robots. Triple Ring overcame these hurdles by employing first-principles thinking and creative engineering.
Outcomes
The partnership led to an innovative prototype design with 4 degrees of freedom that served as the basis of AiM Medical Robotics’ industry-first robotics platform.
Triple Ring Value Proposition
- Experience in designing multi-axis robots for multiple applications
- Deep expertise in the physics of MRI
- Demonstrated capabilities in designing MRI-compatible systems and components
- Rapid design, prototyping, and production to meet tight timelines and funding milestones
MRI-Compatible Robotics
Challenge
AiM Medical Robotics required a portable robotic system to perform neurosurgery simultaneous with magnetic resonance imaging (MRI). MRI relies on incredibly strong magnetic fields to produce detailed images of the inside of the human body. These strong magnetic fields prohibit the use of ferromagnetic materials that are found in typical robotic hardware. Furthermore, MRI systems feature a small bore around the patient table which severely restricts the form factor of potential robots. Triple Ring overcame these hurdles by employing first-principles thinking and creative engineering.
Outcomes
The partnership led to an innovative prototype design with 4 degrees of freedom that served as the basis of AiM Medical Robotics’ industry-first robotics platform.
Triple Ring Value Proposition
- Experience in designing multi-axis robots for multiple applications
- Deep expertise in the physics of MRI
- Demonstrated capabilities in designing MRI-compatible systems and components
- Rapid design, prototyping, and production to meet tight timelines and funding milestones
Client
AiM Medical Robotics
Practice Areas
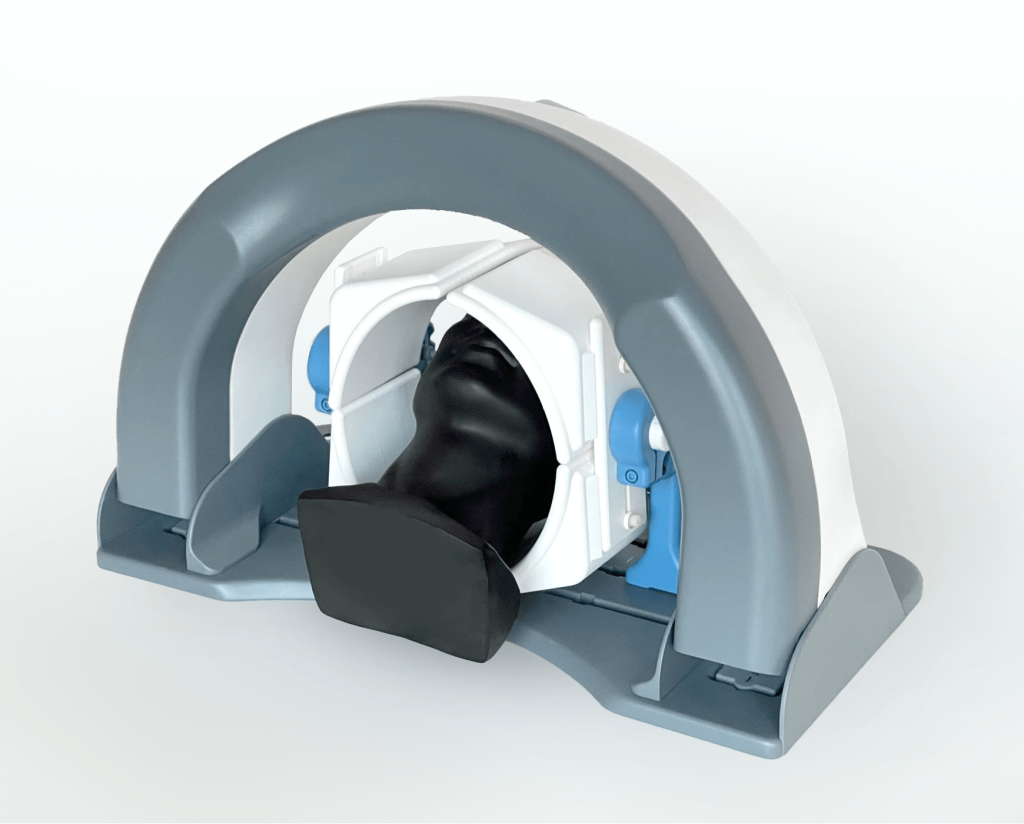
Background
AiM Medical Robotics chose to work with Triple Ring due to our deep scientific knowledge of the principles of MRI, our extensive experience engineering complex robotics, and our ability to develop creative solutions to complicated, constrained problems. Because of this, Triple Ring was able to deliver a design for AiM Medical Robotics that met the stringent requirements of MRI-compatibility.

Upright Radiation Therapy
Client
Leo Cancer Care
Practice Areas
Challenge
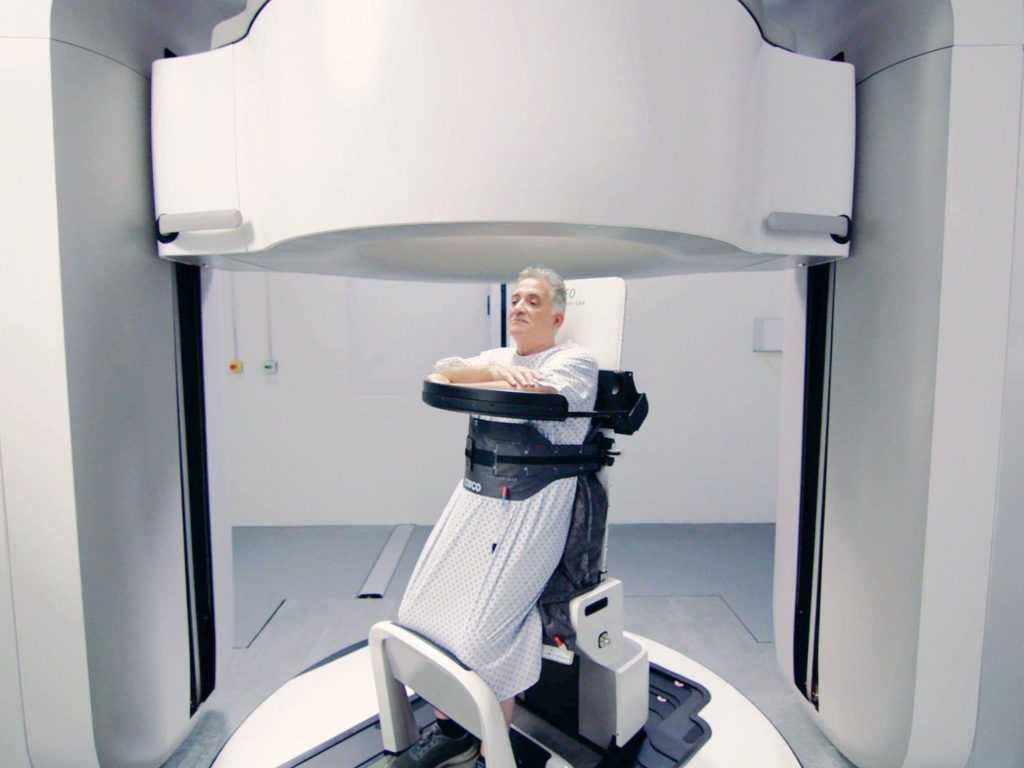
Leo Cancer Care partnered with Triple Ring to design a computed tomography (CT) imaging system for a novel radiation therapy concept that drives better patient outcomes and improved patient experience. Leo Cancer Care is a bold innovator in radiation therapy, developing breakthrough products that treat patients in a more natural, seated position. The system images and positions patients for radiotherapy in one device with the patient upright, improving accuracy while increasing comfort and lowering stress – all contributing factors to better outcomes. Triple Ring collaborated with Leo Cancer Care to design a gantry and CT scanner which scans patients in upright and lying down positions. This concept is central to the platform, making large rotating gantries for the radiotherapy source obsolete, and enabling a fixed beam, slow patient rotation strategy. Leo Cancer Care and Triple Ring together overcame multiple design and technical challenges to transform this novel concept into a safe, accurate, and patient-centric platform.
Outcomes
The Leo Cancer Care and Triple Ring collaboration led to the creation of an innovative gantry design that featured upright radiation therapy as a new standard of care.
Triple Ring Value Proposition
- Experience designing large multi-axis robotics
- Deep expertise in the physics of radiotherapy delivery devices
- Mechanical design of robust and reliable capital equipment to ISO13485 standards
- Advanced simulation and modeling to streamline system design, development, and test
- Shared sense of urgency to meet aggressive schedules and assure funding milestones
Upright Radiation Therapy
Challenge
Leo Cancer Care partnered with Triple Ring to design a computed tomography (CT) imaging system for a novel radiation therapy concept that drives better patient outcomes and improved patient experience. Leo Cancer Care is a bold innovator in radiation therapy, developing breakthrough products that treat patients in a more natural, seated position. The system images and positions patients for radiotherapy in one device with the patient upright, improving accuracy while increasing comfort and lowering stress – all contributing factors to better outcomes. Triple Ring collaborated with Leo Cancer Care to design a gantry and CT scanner which scans patients in upright and lying down positions. This concept is central to the platform, making large rotating gantries for the radiotherapy source obsolete, and enabling a fixed beam, slow patient rotation strategy. Leo Cancer Care and Triple Ring together overcame multiple design and technical challenges to transform this novel concept into a safe, accurate, and patient-centric platform.
Outcomes
The Leo Cancer Care and Triple Ring collaboration led to the creation of an innovative gantry design that featured upright radiation therapy as a new standard of care.
Triple Ring Value Proposition
- Experience designing large multi-axis robotics
- Deep expertise in the physics of radiotherapy delivery devices
- Mechanical design of robust and reliable capital equipment to ISO13485 standards
- Advanced simulation and modeling to streamline system design, development, and test
- Shared sense of urgency to meet aggressive schedules and assure funding milestones
Client
Leo Cancer Care
Practice Areas

Background
Leo Cancer Care chose to work with Triple Ring because of our deep scientific knowledge in energy delivery and medical imaging matched with our proven success in building high-precision mechanical systems for clinical validation and market entry. Our work with Leo Cancer required close collaboration and tight communication to integrate multiple subsystems and produce a high quality, efficacious system.

Next-Generation Equine Imaging
Client
Prisma Imaging
Practice Areas
Challenge
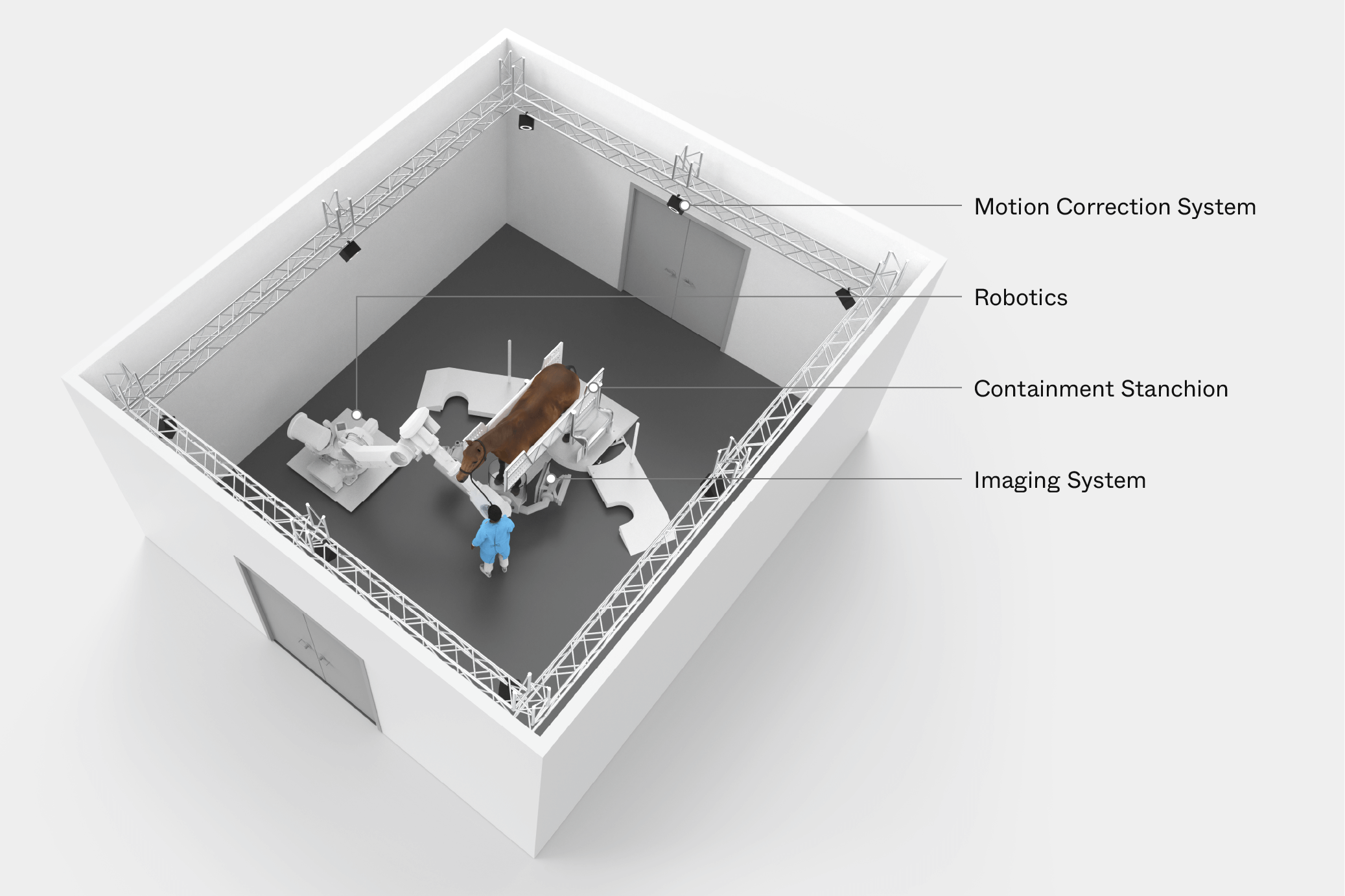
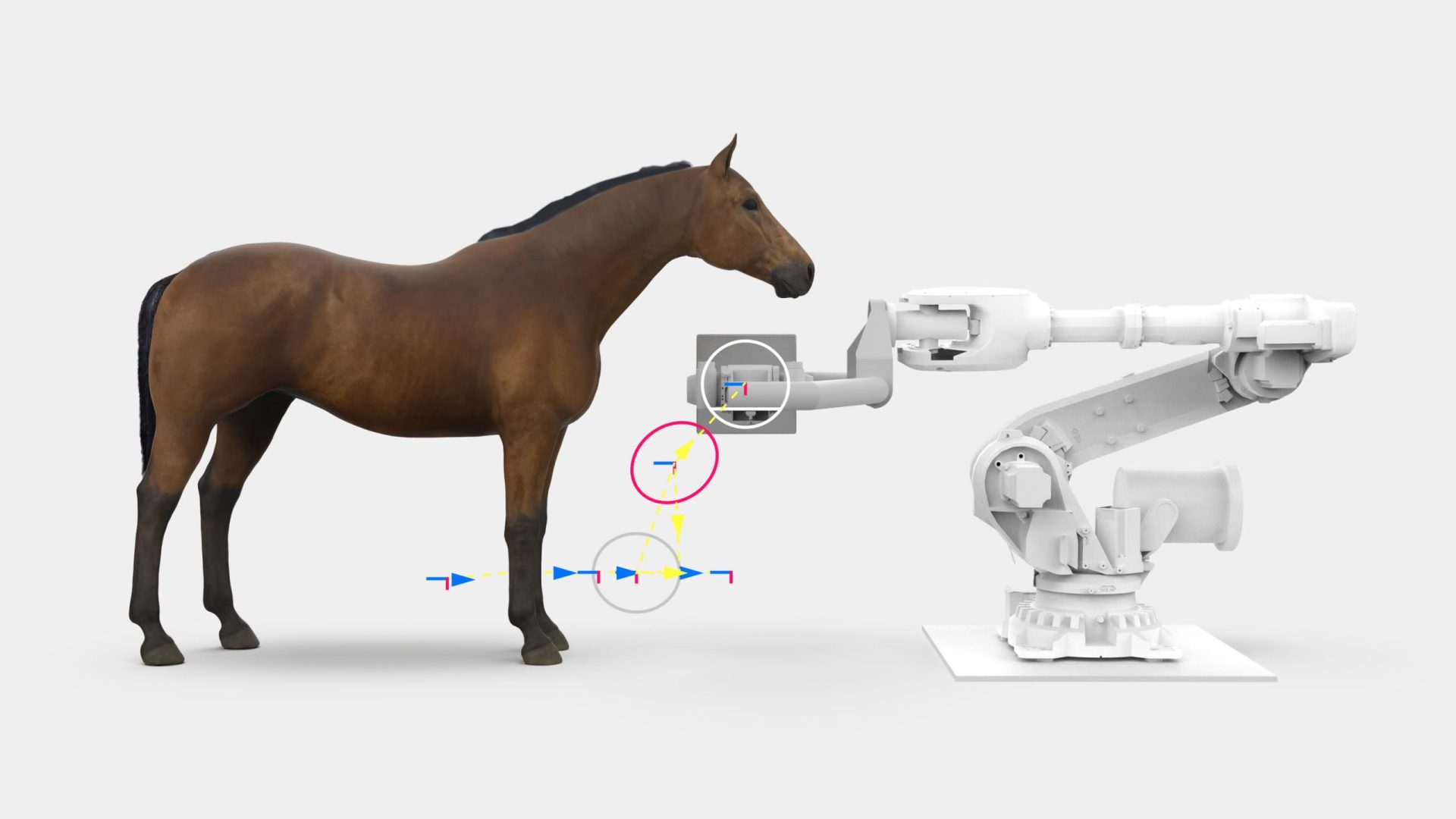
Prisma Imaging partnered with Triple Ring Technologies to design, model, build, and test a functional prototype equine CT scanner for imaging on live animals in a veterinary clinic setting. Prisma Imaging invented a next generation large animal CT imaging system capable of generating high quality images in a vastly safer workflow for equine patients. This complex prototype system included a robotic gantry, motion capture and correction, X-ray imaging, radiation safety, and CT reconstruction engine. Such an extensive system required Triple Ring to leverage its cross-disciplinary expertise and implement a top-down systems engineering approach.
Outcomes
Triple Ring delivered a gantry-mounted CT imaging system, which enabled live-animal imaging at our headquarters in California on horses that were consciously sedated, not anesthetized. With its integrated cutting-edge motion tracking subsystem and correction algorithms, the system performed equivalently to current standard tabletop technology, even in the presence of natural patient movement. The X-ray system was supported by a robust mechanical subsystem and the combined motion/imaging system was carefully controlled by custom software/hardware.
Triple Ring Value Proposition
- Fully outsourced engineering services, from conception to assembly
- World class image reconstruction architecture and algorithm development capabilities
- Hands-on experience assembling complex hardware
- Deep expertise making complicated systems work, with deliberate and careful subsystem and integration testing
- Flexible and customizable lab space to support exotic applications, such as live-animal imaging
Next-Generation Equine Imaging
Challenge
Prisma Imaging partnered with Triple Ring Technologies to design, model, build, and test a functional prototype equine CT scanner for imaging on live animals in a veterinary clinic setting. Prisma Imaging invented a next generation large animal CT imaging system capable of generating high quality images in a vastly safer workflow for equine patients. This complex prototype system included a robotic gantry, motion capture and correction, X-ray imaging, radiation safety, and CT reconstruction engine. Such an extensive system required Triple Ring to leverage its cross-disciplinary expertise and implement a top-down systems engineering approach.
Outcomes
Triple Ring delivered a gantry-mounted CT imaging system, which enabled live-animal imaging at our headquarters in California on horses that were consciously sedated, not anesthetized. With its integrated cutting-edge motion tracking subsystem and correction algorithms, the system performed equivalently to current standard tabletop technology, even in the presence of natural patient movement. The X-ray system was supported by a robust mechanical subsystem and the combined motion/imaging system was carefully controlled by custom software/hardware.
Triple Ring Value Proposition
- Fully outsourced engineering services, from conception to assembly
- World class image reconstruction architecture and algorithm development capabilities
- Hands-on experience assembling complex hardware
- Deep expertise making complicated systems work, with deliberate and careful subsystem and integration testing
- Flexible and customizable lab space to support exotic applications, such as live-animal imaging
Client
Prisma Imaging
Practice Areas


Background
The system architecture developed and implemented by Triple Ring enabled Prisma’s system to be the only CT imaging technology that can access an equine patient’s entire anatomy without the use of anesthesia. Triple Ring was chosen for this aggressive project because of our deep technical knowledge in the diverse science and engineering disciplines required for success. No animals were harmed during testing.
Intra-Operative Tissue Pathology
Client
BlackLight Surgical
Practice Areas
Challenges
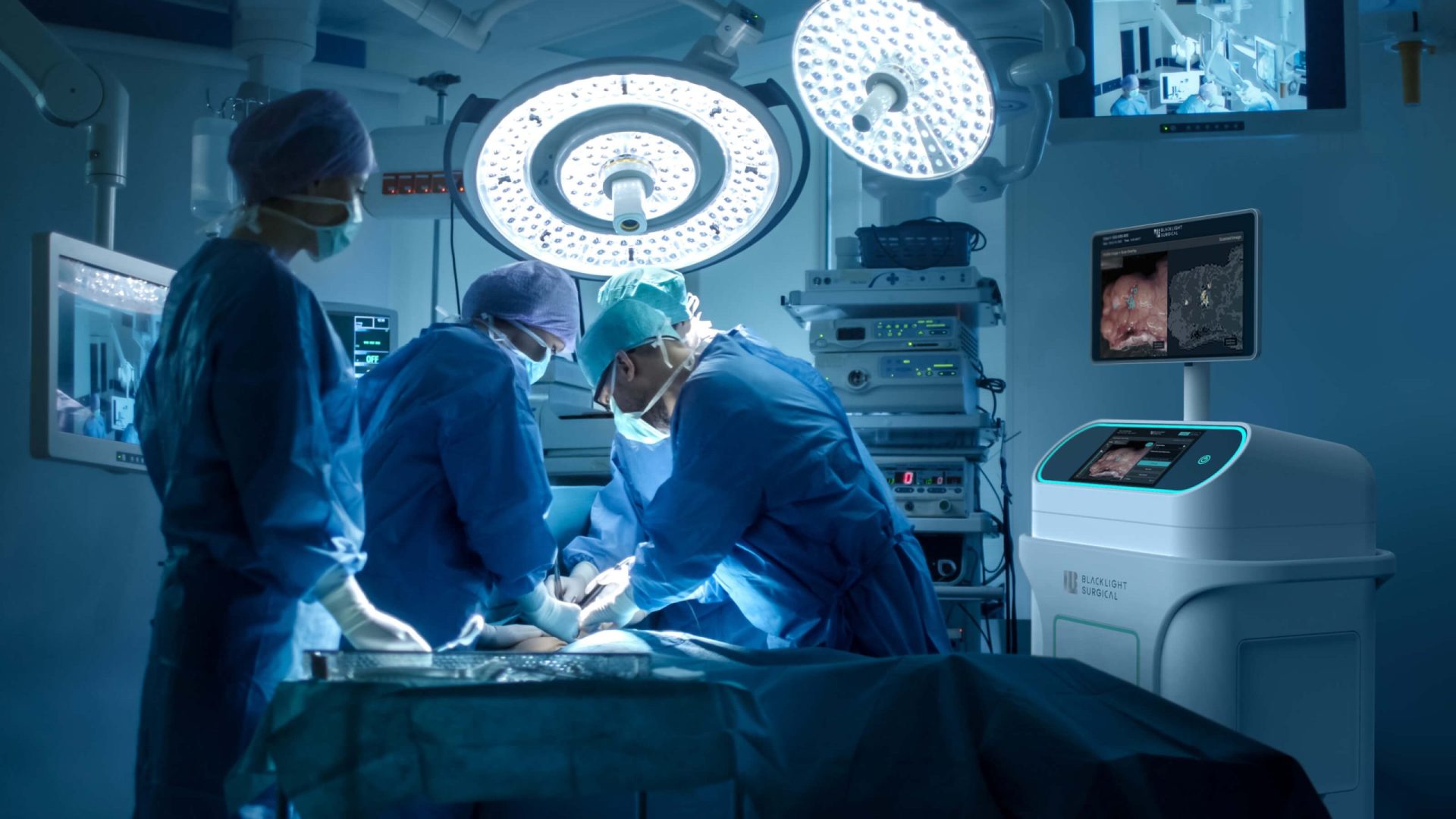
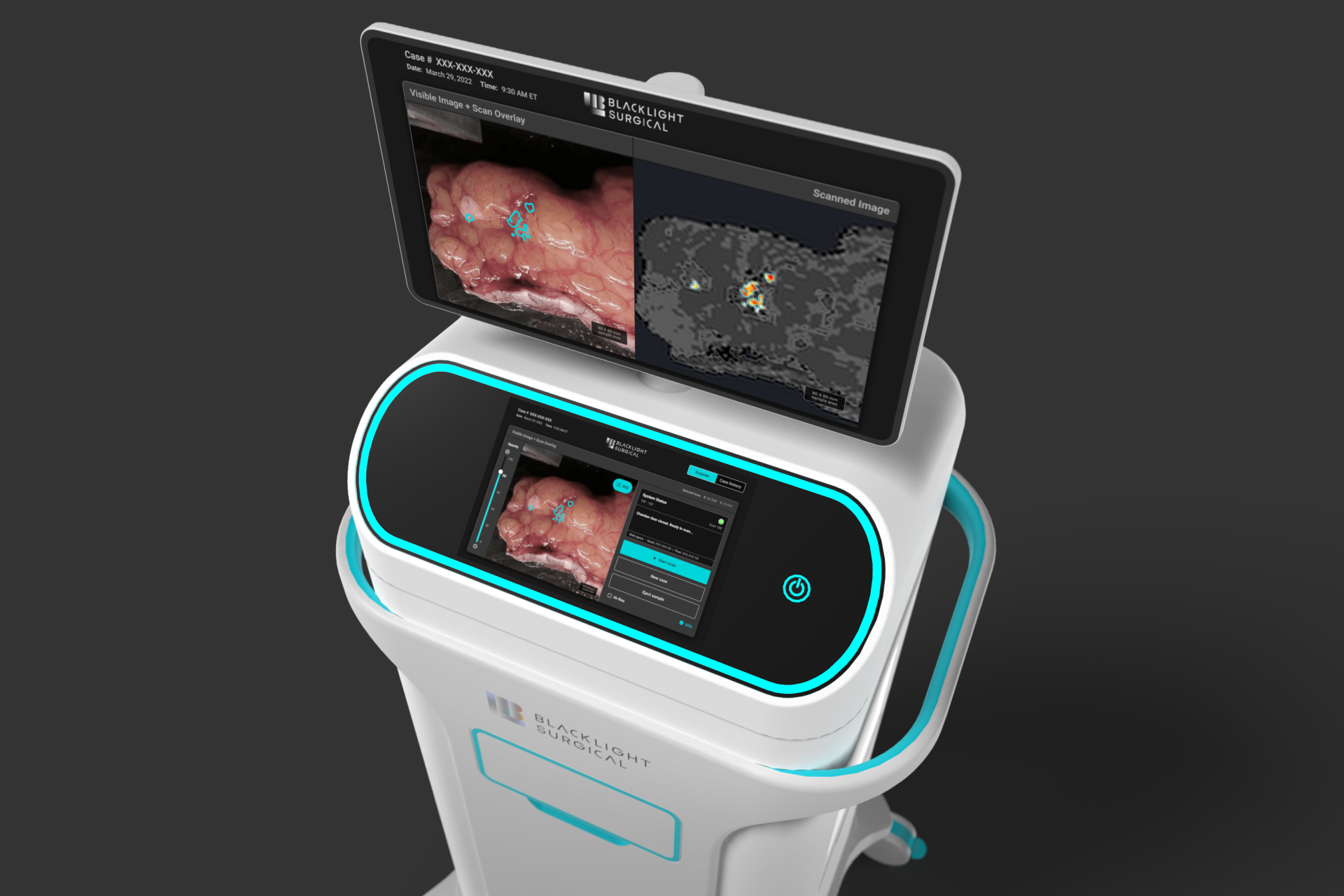
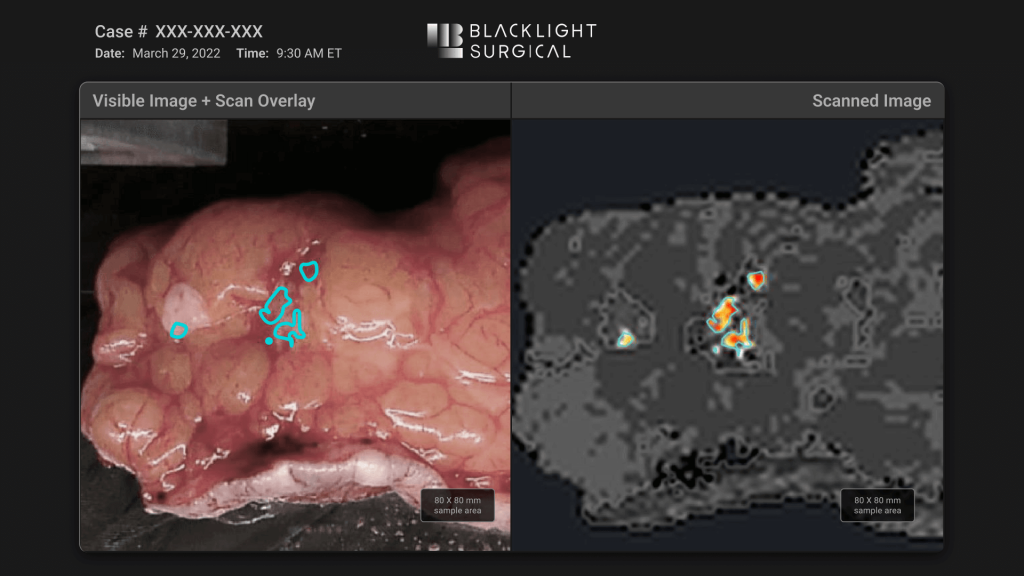
Triple Ring Technologies was tasked with designing, integrating, testing, and delivering an innovative high-speed laser-based intra-operative biochemical imaging platform. The system leveraged picosecond pulsing laser technology for intra-operative tissue analysis. A user-centric design and workflow facilitated by machine learning successfully enabled real-time clinical decision making in the surgery suite.
Outcomes
Through Triple Ring’s proven product design and development process, Blacklight Surgical’s breakthrough imaging platform was built and delivered to support critical clinical studies. The system was designed to ISO13485 standards and fully documented within a Quality Management System (QMS) established by Triple Ring and transferred to Black Light Surgical.
Triple Ring Value Proposition
- Multidisciplinary science and engineering teams capable of design and development, integration, verification, test, and system build
- Included Engineering (Mechanical, Electrical, Software, Systems, BioMedical,and Test), Optical Science, Machine Learning, Industrial Design, Quality Management, and system assembly.
- Efficient design process featuring extensive modeling and complex simulations to derive requirements and guide prototyping.
Intra-Operative Tissue Pathology
Challenges
Triple Ring Technologies was tasked with designing, integrating, testing, and delivering an innovative high-speed laser-based intra-operative biochemical imaging platform. The system leveraged picosecond pulsing laser technology for intra-operative tissue analysis. A user-centric design and workflow facilitated by machine learning successfully enabled real-time clinical decision making in the surgery suite.
Outcomes
Through Triple Ring’s proven product design and development process, Blacklight Surgical’s breakthrough imaging platform was built and delivered to support critical clinical studies. The system was designed to ISO13485 standards and fully documented within a Quality Management System (QMS) established by Triple Ring and transferred to Black Light Surgical.
Triple Ring Value Proposition
- Multidisciplinary science and engineering teams capable of design and development, integration, verification, test, and system build
- Included Engineering (Mechanical, Electrical, Software, Systems, BioMedical,and Test), Optical Science, Machine Learning, Industrial Design, Quality Management, and system assembly.
- Efficient design process featuring extensive modeling and complex simulations to derive requirements and guide prototyping.
Client
BlackLight Surgical
Practice Areas
Background
BlackLight Surgical has harnessed the principles of Time-Resolved Laser Induced Fluorescence Spectroscopyto innovate within intra-operative pathology workflows. Collaborating with Triple Ring, they have created a new biochemical imaging platform that identifies margins between normal and disease tissues inside the surgical suite, in real-time, without labels. With our full teams of scientists, engineers, designers, and assembly technicians, Triple Ring was ideally suited to translate Blackight Surgical’s breakthrough technology to the clinic.

Transdermal GFR Measurement
Client
MediBeacon
Practice Areas
Challenge
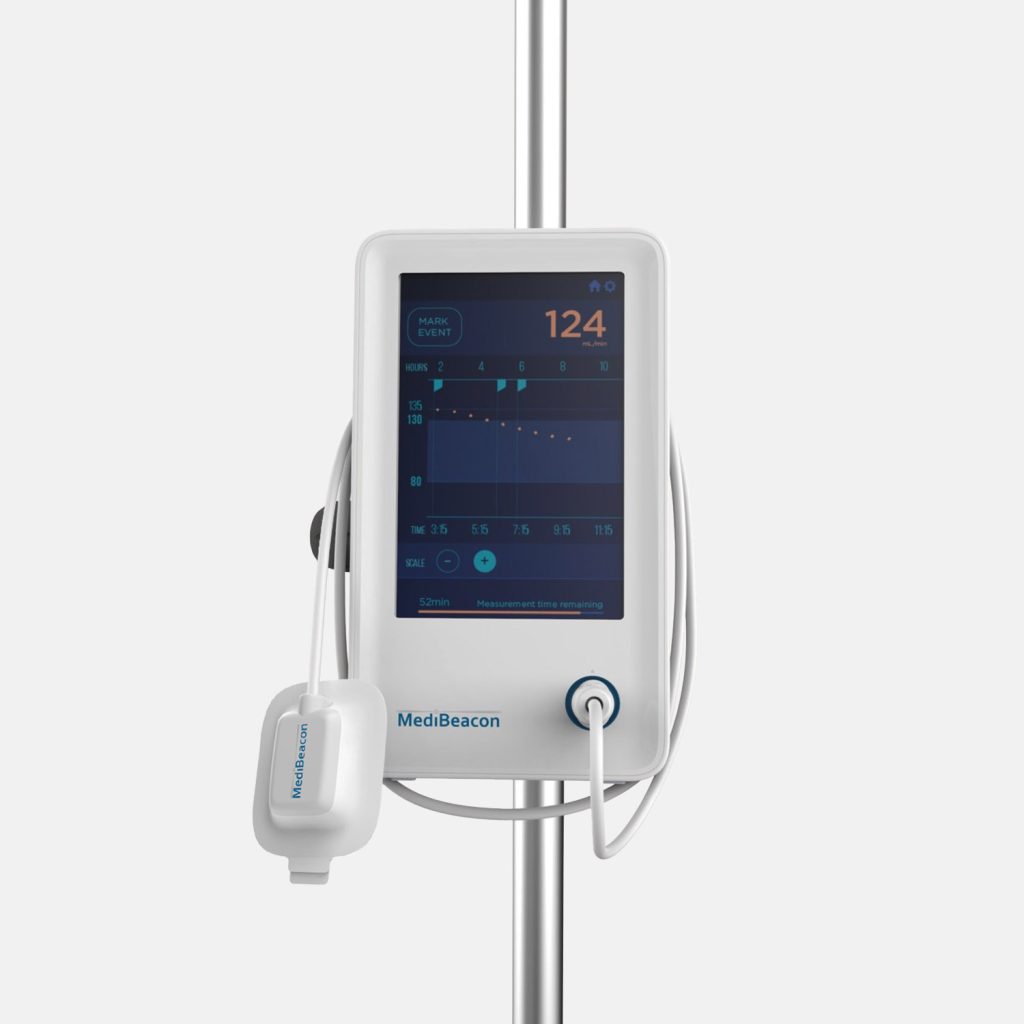
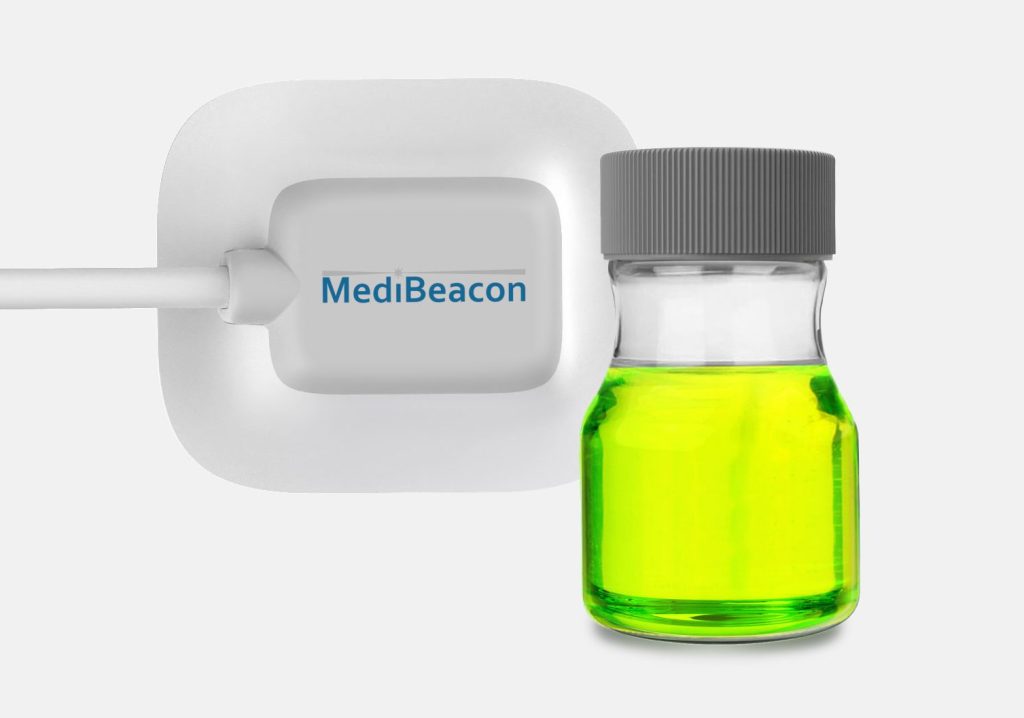
MediBeacon required expert assistance to develop a photonics-based transdermal detection technology to monitor intravenously injected fluorescent tracer agents and provide clinically actionable measures of kidney function. MediBeacon has brought combination products into the clinic which match fluorescent tracers with innovative detection systems to provide accurate physiological measurements in real-time. Triple Ring, in collaboration with MediBeacon, designed and delivered a non-invasive, light-based wearable detection system as a critical component of their glomerular filtration rate (GFR) monitoring product. The detectors were designed and developed to meet strict product requirements of sensitivity, accuracy, cost, comfort, and ease of use.
Outcomes
Fully integrated wearable GFR detectors were developed under ISO13485 design controls and delivered for critical clinical trials.
Triple Ring Value Proposition
- Highly relevant expertise in light-based tissue analysis
- Advanced modeling and simulations to speed design process
- Program structure that included skin in the game
- Deep experience in meeting critical milestones for venture funding
Transdermal GFR Measurement
Challenge
MediBeacon required expert assistance to develop a photonics-based transdermal detection technology to monitor intravenously injected fluorescent tracer agents and provide clinically actionable measures of kidney function. MediBeacon has brought combination products into the clinic which match fluorescent tracers with innovative detection systems to provide accurate physiological measurements in real-time. Triple Ring, in collaboration with MediBeacon, designed and delivered a non-invasive, light-based wearable detection system as a critical component of their glomerular filtration rate (GFR) monitoring product. The detectors were designed and developed to meet strict product requirements of sensitivity, accuracy, cost, comfort, and ease of use.
Outcomes
Fully integrated wearable GFR detectors were developed under ISO13485 design controls and delivered for critical clinical trials.
Triple Ring Value Proposition
- Highly relevant expertise in light-based tissue analysis
- Advanced modeling and simulations to speed design process
- Program structure that included skin in the game
- Deep experience in meeting critical milestones for venture funding
Client
MediBeacon
Practice Areas
Background
MediBeacon selected Triple Ring as their development partner for our multidisciplinary science and engineering teams. Triple Ring’s industry leading capabilities in biophotonics and combination product development, along with our ability to collaborate closely and share risk, made us an optimal partner for MediBeacon.


Veterinary Vital Sign Monitor
Client
One Health Group
Practice Areas
Challenge
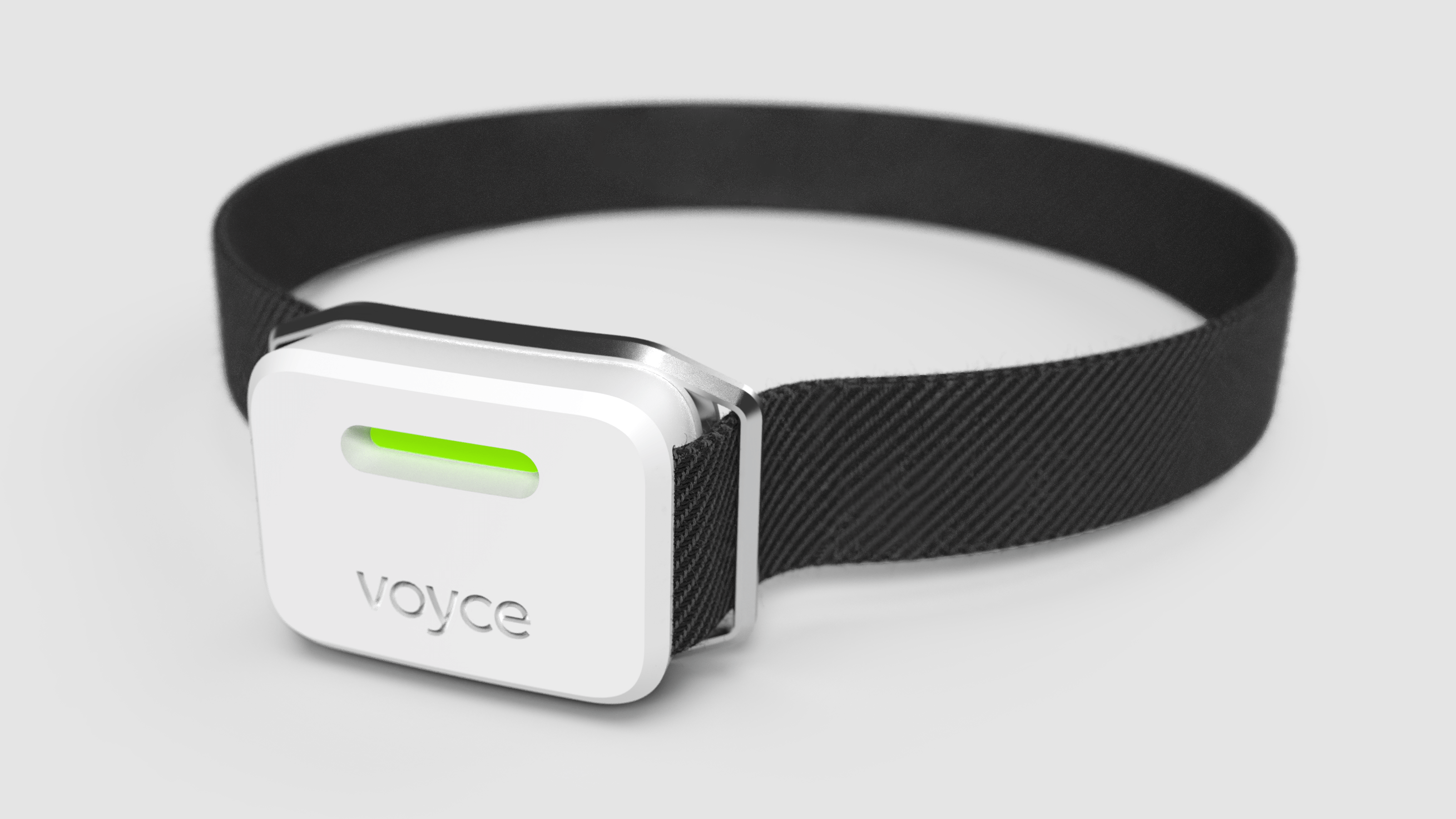
To accurately measure biometric data and improve outcomes in the veterinary setting, Triple Ring Technologies developed a noninvasive, remote digital monitoring device for One Health Group. Triple Ring’s experts designed a wearable physiological monitor that improved patient comfort, achieved long-term continuous monitoring, provided real-time feedback and alerts, and was cost-effective and easy to use.
Outcomes
The robust functional prototypes generated high quality physiologic data that supported One Health Group’s value proposition and enabled expanded partnerships with strategic investors in the veterinary health industry.
Value Propositions
- Client outsourced R&D to Triple Ring which provided expertise tailored for a virtual startup company
- Deep and relevant clinical and technical domain knowledge
- Concept to clinical validation services
- Urgency to drive value and support fund raising
Veterinary Vital Sign Monitor
Challenge
To accurately measure biometric data and improve outcomes in the veterinary setting, Triple Ring Technologies developed a noninvasive, remote digital monitoring device for One Health Group. Triple Ring’s experts designed a wearable physiological monitor that improved patient comfort, achieved long-term continuous monitoring, provided real-time feedback and alerts, and was cost-effective and easy to use.
Outcomes
The robust functional prototypes generated high quality physiologic data that supported One Health Group’s value proposition and enabled expanded partnerships with strategic investors in the veterinary health industry.
Value Propositions
- Client outsourced R&D to Triple Ring which provided expertise tailored for a virtual startup company
- Deep and relevant clinical and technical domain knowledge
- Concept to clinical validation services
- Urgency to drive value and support fund raising
Client
One Health Group
Practice Areas
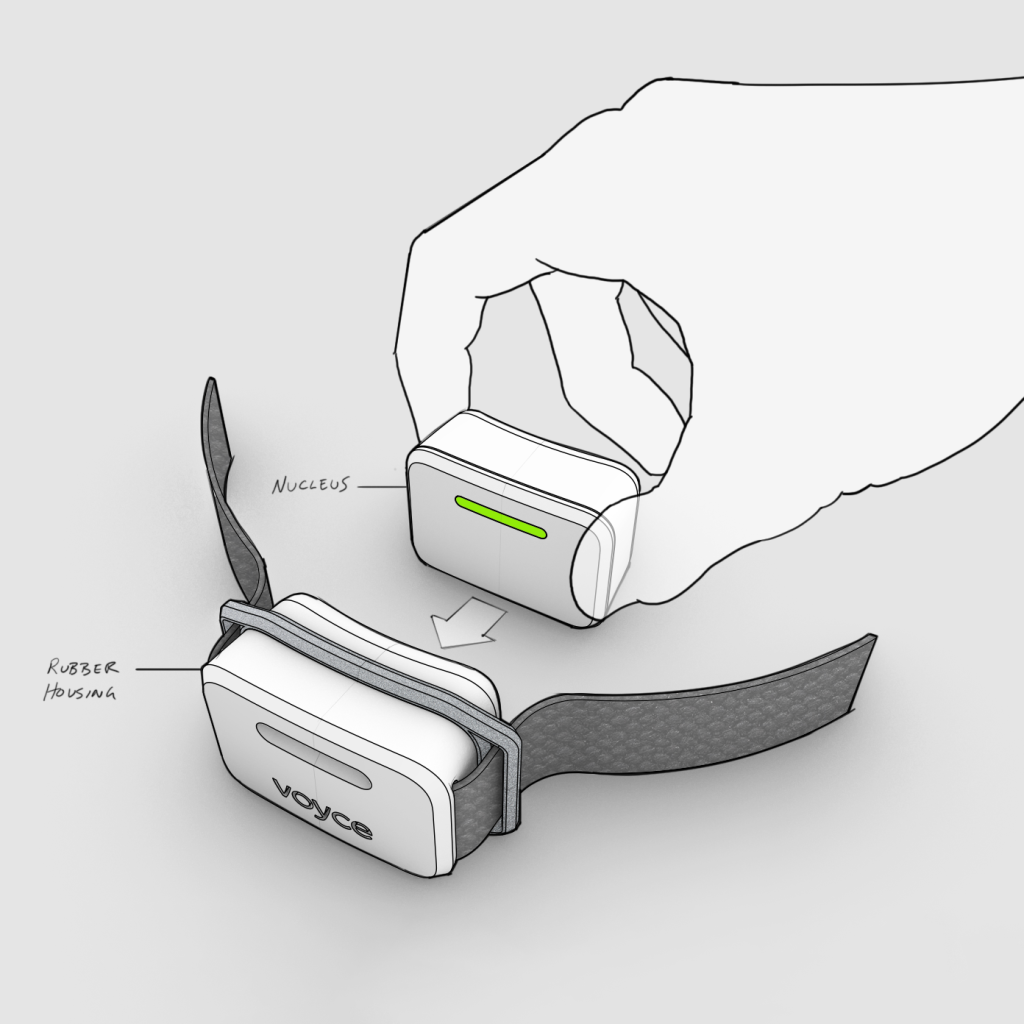

Background
One Health’s business model depended on a lean organization. The company required a close outsourced partner to perform all their R&D. One Health’s system is a complex device requiring a multidisciplinary team to design, build and test. Through concept development, industrial design and prototyping, Triple Ring ran a rapid feasibility demonstration process to de-risk critical elements of the product design and prove functionality.
Our technology partnership with Triple Ring will enable us to much more quickly bring our patented mobile health monitoring technology to market and will support our goals as we build on our noncontact intelligent biometric sensing Voyce platform, bringing new, innovative physiological monitoring capabilities to support in-situ and real-time animal health care
Triple Ring is pleased to partner with One Health Group to develop the next version of their exciting platform for next-generation remote animal health monitoring. We believe in One Health Group and are taking a stake in the company, showing our support for their technology and the potential for the Voyce platform

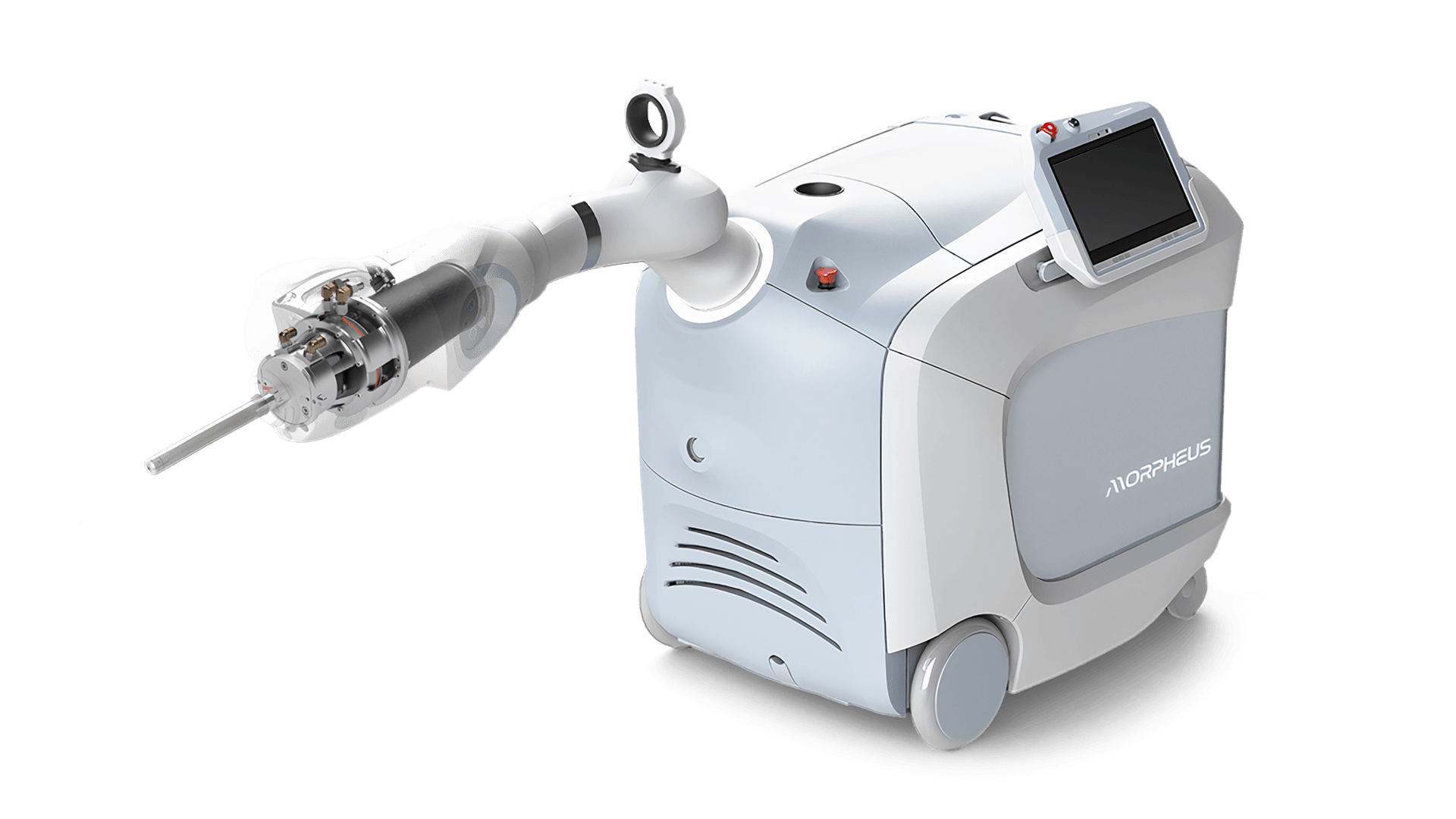
Robotic Radiation Therapy
Client
Empyrean
Practice Areas
Challenge
Triple Ring Technologies designed and developed a novel intra-operative radiation therapy device for Empyrean Medical Systems and facilitated submission of a complete design package to FDA for 510(k) clearance. The compact, mobile, robotically guided, low-energy, intra-operative radiation therapy device was designed with the patient in mind and with a tight focus on usability.
Outcomes
A full system was designed, integrated, verified, clinically validated, and submitted to FDA for marketing clearance. Post 510(k), the device design was transferred to manufacture and launched into the market.
Triple Ring Value Proposition
- Innovative custom x-ray source design for true 3D beam directionality
- Valuable intellectual property created
- Advanced modeling and Monte Carlo simulation to speed design process
- System integration and transfer to manufacture services
- Strong track record of FDA clearance for device designs
Robotic Radiation Therapy
Challenge
Triple Ring Technologies designed and developed a novel intra-operative radiation therapy device for Empyrean Medical Systems and facilitated submission of a complete design package to FDA for 510(k) clearance. The compact, mobile, robotically guided, low-energy, intra-operative radiation therapy device was designed with the patient in mind and with a tight focus on usability.
Outcomes
A full system was designed, integrated, verified, clinically validated, and submitted to FDA for marketing clearance. Post 510(k), the device design was transferred to manufacture and launched into the market.
Triple Ring Value Proposition
- Innovative custom x-ray source design for true 3D beam directionality
- Valuable intellectual property created
- Advanced modeling and Monte Carlo simulation to speed design process
- System integration and transfer to manufacture services
- Strong track record of FDA clearance for device designs
Client
Empyrean
Practice Areas


Background
Empryean Medical and Triple Ring collaborated on the Morpheus System throughout the product development life cycle from concept to market launch. Triple Ring’s contributions included design and development of critical subsystems (x-ray source and beam steering electronics, for examples) to system integration, verification and test, clinical validation, and FDA submission. Triple Ring was chosen as Empyrean’s co-development partner due to our vast experience commercializing complex energy delivery technology for a range of clinical applications.