The United States currently leads the world in government support for non-military research and development (R&D), especially support for work that directly relates to health and human development. A focal point for such investments to date in biomedical research has been the National Institutes of Health (NIH), receiving approximately $41.7 billion in fiscal year 2020. Approximately 9% of this funding is spent annually on internal R&D projects (intramural research) utilizing the work of about 9,000 scientists. The other funding is largely utilized to support the work of 35,000 non-government investigators (extramural research) at various colleges and universities in the U.S. and abroad as well as corporate research undertaken at small businesses.
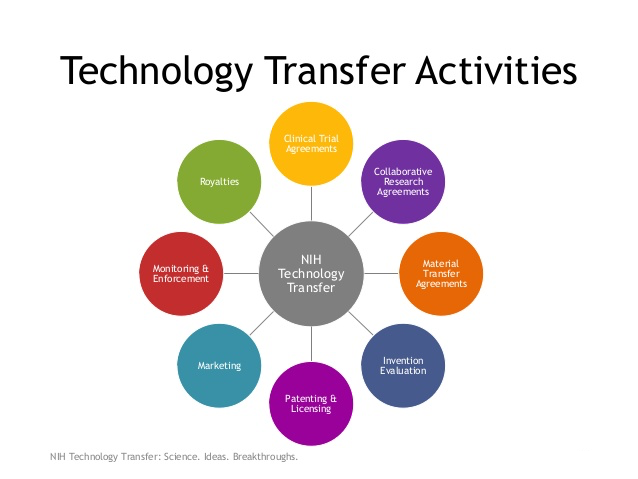
Whether internal or externally based, the biomedical research performed has led to a large variety of novel basic and clinical research discoveries – all of which generally require commercial partners in order to develop them into products for hospital, physician or patient use. Thus, the NIH needs and actively seeks partners and licensees to help develop and commercialize its research into products to help fulfill its mission as a healthcare agency.
The presentation will examine how the NIH technology transfer program works to meet these goals as well as offering suggestions and practical advice for organizations seeking new product ideas or looking to interact with NIH scientists in order to start or expand their businesses. With over 700 products from these license agreements currently on the market, licensing, collaboration, funding or contract activities with NIH cannot be overlooked as part of growth strategies for biomedical firms.
Images are licensed under Creative Commons License.

