IVD Platform Refresh
Client
Fortune 500 IVD Company
Practice Areas
Challenge
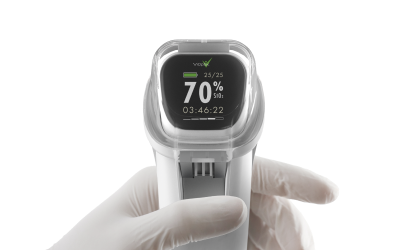
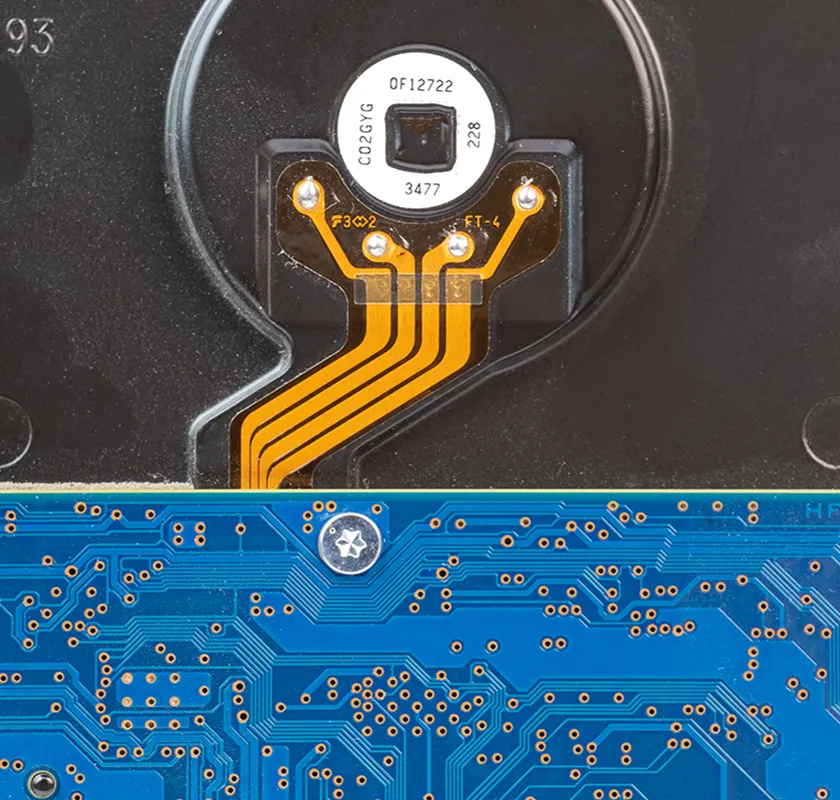
Our client faced the obsolescence of critical components within a legacy IVD product platform, including single-board computers and microcontrollers. A lack of original firmware source code and the sunsetting of institutional knowledge required extensive reverse engineering before updates could be designed and implemented. Adding further complexity, Triple Ring was tasked with modernizing the product to maintain market competitiveness yet demonstrate equivalence to fulfill the criteria of a Special 510(k) regulatory pathway.
Outcomes
A full system design and regulatory package was delivered for a refreshed IVD product with demonstrated equivalency to its FDA-cleared predecessor. Triple Ring replaced obsolete components, reverse-engineered and migrated existing software to a new OS, updated subassemblies, and developed new firmware compatible with legacy systems. The refreshed system featured modern industrial design, met manufacturing cost targets, and integrated seamlessly with existing manufacturing processes, ensuring uninterrupted production.
Value Propositions
- Multidisciplinary teams skilled in reverse engineering and future-proofing
- Software porting expertise
- In-depth knowledge of clinical IVD devices and relevant regulatory requirements
- Strong collaboration with client manufacturing and service teams
- Industrial Design capabilities to modernize user experience
IVD Platform Refresh
Challenge
Our client faced the obsolescence of critical components within a legacy IVD product platform, including single-board computers and microcontrollers. A lack of original firmware source code and the sunsetting of institutional knowledge required extensive reverse engineering before updates could be designed and implemented. Adding further complexity, Triple Ring was tasked with modernizing the product to maintain market competitiveness yet demonstrate equivalence to fulfill the criteria of a Special 510(k) regulatory pathway.
Outcomes
A full system design and regulatory package was delivered for a refreshed IVD product with demonstrated equivalency to its FDA-cleared predecessor. Triple Ring replaced obsolete components, reverse-engineered and migrated existing software to a new OS, updated subassemblies, and developed new firmware compatible with legacy systems. The refreshed system featured modern industrial design, met manufacturing cost targets, and integrated seamlessly with existing manufacturing processes, ensuring uninterrupted production.
Value Propositions
- Multidisciplinary teams skilled in reverse engineering and future-proofing
- Software porting expertise
- In-depth knowledge of clinical IVD devices and relevant regulatory requirements
- Strong collaboration with client manufacturing and service teams
- Industrial Design capabilities to modernize user experience
Client
Fortune 500 IVD Company
Practice Areas

Background
A large multinational corporation specializing in In Vitro Diagnostic (IVD) devices engaged with Triple Ring to refresh and future-proof a legacy product line facing component and software obsolescence. Triple Ring executed an accelerated program to modernize the platform while holding production costs steady and demonstrating equivalent performance.