Transdermal GFR Measurement
Client
MediBeacon
Practice Areas
Challenge
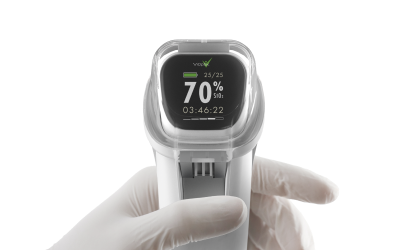
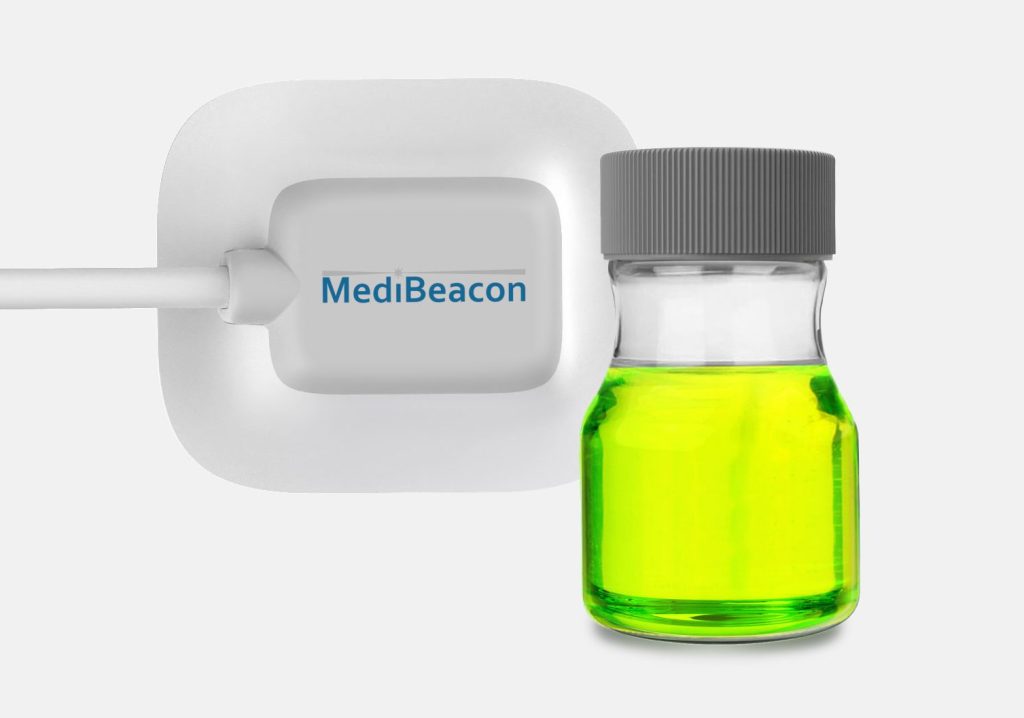
MediBeacon required expert assistance to develop a photonics-based transdermal detection technology to monitor intravenously injected fluorescent tracer agents and provide clinically actionable measures of kidney function. MediBeacon has brought combination products into the clinic which match fluorescent tracers with innovative detection systems to provide accurate physiological measurements in real-time. Triple Ring, in collaboration with MediBeacon, designed and delivered a non-invasive, light-based wearable detection system as a critical component of their glomerular filtration rate (GFR) monitoring product. The detectors were designed and developed to meet strict product requirements of sensitivity, accuracy, cost, comfort, and ease of use.
Outcomes
Fully integrated wearable GFR detectors were developed under ISO13485 design controls and delivered for critical clinical trials.
Triple Ring Value Proposition
- Highly relevant expertise in light-based tissue analysis
- Advanced modeling and simulations to speed design process
- Program structure that included skin in the game
- Deep experience in meeting critical milestones for venture funding
Transdermal GFR Measurement
Challenge
MediBeacon required expert assistance to develop a photonics-based transdermal detection technology to monitor intravenously injected fluorescent tracer agents and provide clinically actionable measures of kidney function. MediBeacon has brought combination products into the clinic which match fluorescent tracers with innovative detection systems to provide accurate physiological measurements in real-time. Triple Ring, in collaboration with MediBeacon, designed and delivered a non-invasive, light-based wearable detection system as a critical component of their glomerular filtration rate (GFR) monitoring product. The detectors were designed and developed to meet strict product requirements of sensitivity, accuracy, cost, comfort, and ease of use.
Outcomes
Fully integrated wearable GFR detectors were developed under ISO13485 design controls and delivered for critical clinical trials.
Triple Ring Value Proposition
- Highly relevant expertise in light-based tissue analysis
- Advanced modeling and simulations to speed design process
- Program structure that included skin in the game
- Deep experience in meeting critical milestones for venture funding
Client
MediBeacon
Practice Areas
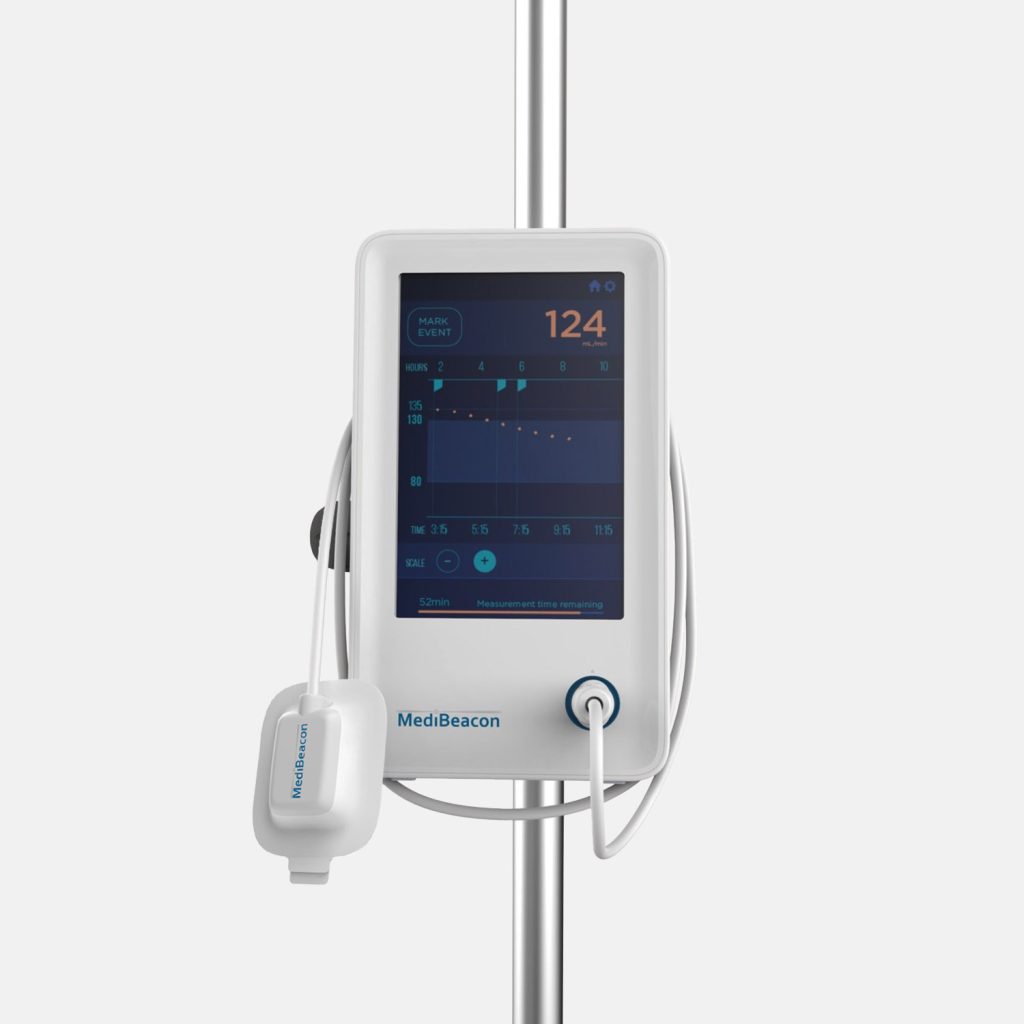
Background
MediBeacon selected Triple Ring as their development partner for our multidisciplinary science and engineering teams. Triple Ring’s industry leading capabilities in biophotonics and combination product development, along with our ability to collaborate closely and share risk, made us an optimal partner for MediBeacon.